Thousands and thousands of over weight or overweight sufferers with coronary heart situations insured below Medicare could get access to a well-liked fat-loss drug, a analyze has identified.
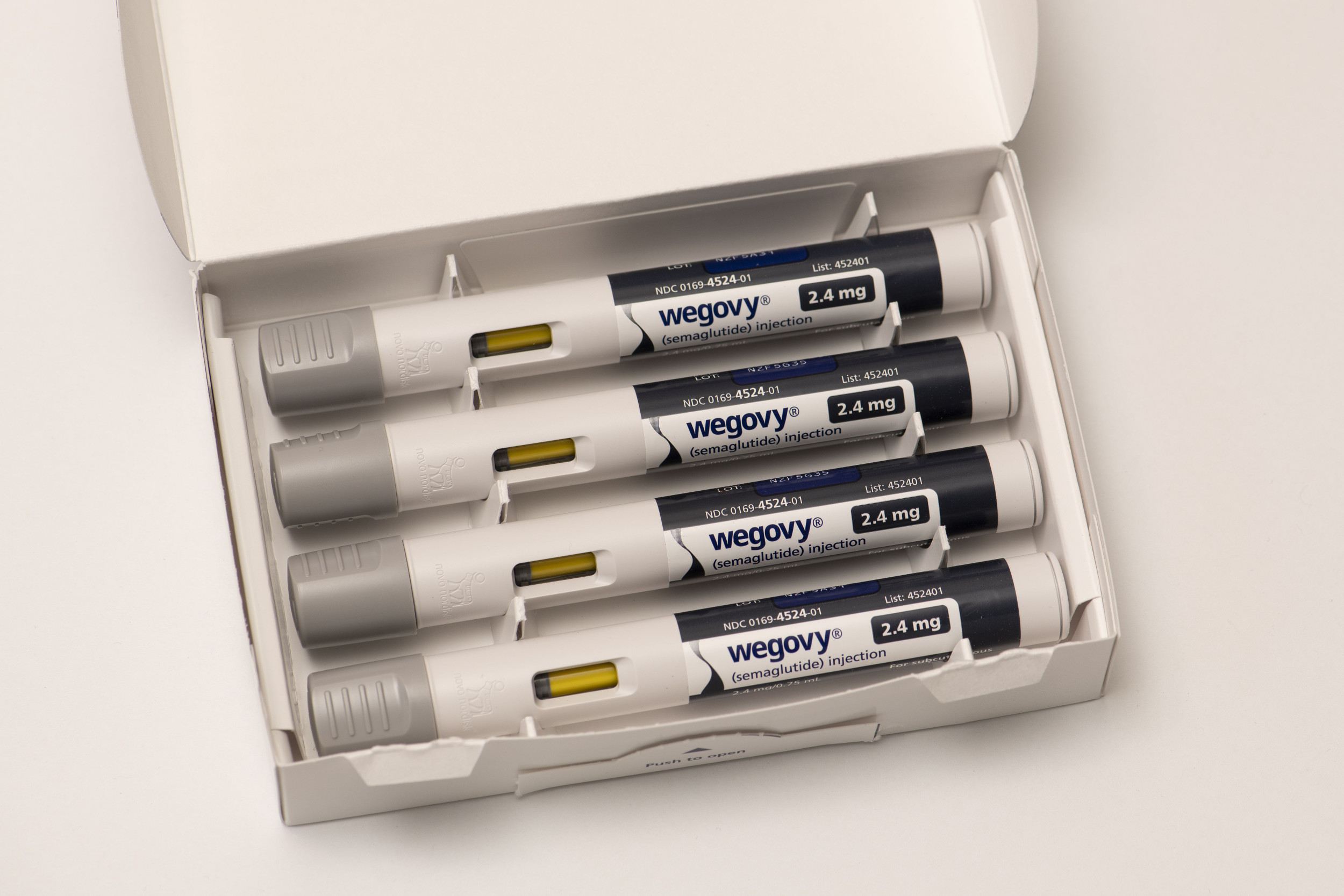
Novo Nordisk’s Wegovy, chemically recognized as semaglutide, was approved by the U.S. Food and Drug Administration past month for lowering the possibility of stroke and coronary heart assault in over weight or overweight grownups who do not have diabetes.
The Fda‘s approval of the new use for Wegovy potentially opens up obtain to the drug for a quarter of men and women on Medicare who are obese or overweight, in accordance to a research released by the Kaiser Spouse and children Foundation (KFF) on Wednesday. Medicare is prohibited by law from covering Wegovy and other medicine for fat decline alone.
The analyze located that an estimated 7 p.c of Medicare beneficiaries, or 3.6 million over-all, experienced established cardiovascular sickness and were obese or overweight in 2020, and they could be suitable for protection of Wegovy for its new indicator. Amongst people people, 1.9 million also experienced diabetes, the KFF review explained, and could have been currently been qualified for Medicare coverage of Wegovy and Novo’s greatly used diabetic issues drug, Ozempic.
Michael Siluk/UCG/Universal Photographs Group via Getty Illustrations or photos
Both belong to a course of drugs referred to as GLP-1 agonists, which were initially created to deal with sort 2 diabetes, but are also hugely powerful pounds-decline medicine.
Due to the fact Wegovy is a self-administered injectable drug, Medicare coverage would be provided beneath drug strategies administered by non-public insurers, recognized as Part D.
The KFF examine said that though some Section D programs have introduced they will begin covering Wegovy in 2024, broader protection in 2025 could be additional probable.
Several Component D strategies might be reluctant to increase protection now owing to the significant price of the drug and the large quantity of individuals eligible for it “considering that they can not regulate their rates mid-calendar year to account for bigger prices associated with use of this drug,” the examine reported.
How the expanded protection of Wegovy will effects Medicare expending will also depend on how a lot of Section D options insert coverage for it and the extent to which the designs utilize constraints like prior authorization, how quite a few individuals who qualify to acquire the drug use it and the negotiated costs paid by programs, the analyze explained.
The U.S. Facilities for Medicare and Medicaid Providers (CMS) issued a memo previous month indicating that Element D plans can include Wegovy now that it has an indicator that is not excluded from Medicare protection.
“CMS has issued direction to Medicare Aspect D ideas stating that anti-weight problems drugs (AOMs) that obtain Fda approval for an extra medically accepted indicator can be considered a Element D drug for that distinct use,” a spokesperson for the federal company instructed Newsweek last month. “Section D coverage is nonetheless not out there for AOMs when applied for persistent fat management in clients who do not have the supplemental medically approved indication, except provided as a supplemental advantage by the Portion D strategy.”
The company has been contacted for additional remark through e mail.
The study also noted that not all Medicare clients suitable to take Wegovy are probable to get it, as some could be set off by the out-of-pocket costs—which could be involving $325 and $430 monthly, just before reaching the $2,000 annual cap—and the probable aspect outcomes of the drug.
The Food and drug administration mentioned the prescribing information for Wegovy consists of a warning to well being about the hazard of thyroid C-cell tumors and that the drug must not be used in clients with a individual or spouse and children historical past of medullary thyroid carcinoma or in patients with a uncommon problem called a number of endocrine neoplasia syndrome kind 2.
The drug also includes warnings for irritation of the pancreas (pancreatitis), gallbladder challenges (which include gallstones), very low blood sugar, acute kidney harm, hypersensitivity reactions, diabetic retinopathy (destruction to the retina), elevated heart price and suicidal behavior or pondering.
A spokesperson for Novo Nordisk formerly informed Newsweek that the business “is continually doing surveillance of the knowledge from ongoing clinical trials and serious-earth use of its goods and collaborates carefully with the authorities to make sure affected person basic safety and sufficient details to health care pros.”
Unheard of Expertise
Newsweek is fully commited to complicated conventional knowledge and obtaining connections in the look for for frequent ground.
Newsweek is committed to challenging common wisdom and discovering connections in the look for for widespread ground.








:quality(85):upscale()/2024/05/29/787/n/24155406/7d9bad8566576bb08f3656.86644031_.jpg)





