Atom-Thick Gold Coating Sparks Scientific ‘Goldene Rush’
Ultrathin gold was achieved with the help of a century-old sword-making technique
Akinbostanci/Getty Images
A glitzy holy grail in materials science has just been attained: scientists have created freestanding, single-atom-thick sheets of gold. This achievement is the first of its kind with any metal atoms, which seem to abhor flatness and typically insist on clustering into droplets or particles.
The method behind the new monolayer metal, dubbed goldene, could “expand the boundaries of what it’s possible to do with materials,” says Lars Hultman, a materials scientist at Linköping University in Sweden and senior author on a new study in Nature Synthesis on the technique. Gold is of particular interest, he adds, because its nanoparticles are already used in electronics, photonics, sensing, biomedicine, and more. The researchers expect that goldene will exhibit its own intriguing suite of new properties, like the single-atom sheets of carbon known as graphene have.
This latest breakthrough builds on previous work in which Hultman and his colleagues embedded gold atoms inside titanium silicon carbide by heating layered films of the materials to about 670 degrees Celsius, causing gold to displace some of the silicon. “The good news was that we had gold layers that were just one atom thick,” Hultman says. “The bad news was that they were stuck inside the host crystal.”
On supporting science journalism
If you’re enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
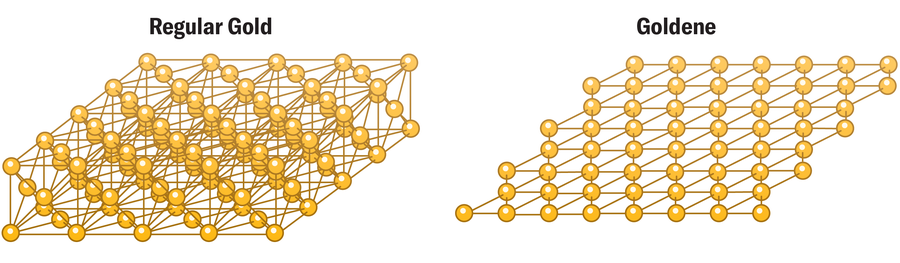
Amanda Montañez; Source: “Synthesis of Goldene Comprising Single-Atom Layer Gold,” by Shun Kashiwaya et al., in Nature Synthesis. Published online April 16, 2024 (reference)
After years of brainstorming how to remove the base material casing—called exfoliation—while preserving the delicate sheets of gold inside, Hultman and his colleagues followed a promising lead with Murakami’s reagent, a solution used in a century-old technique for etching Japanese swords and other metals. But the first attempts to use the reagent were “a total failure,” Hultman says. The gold and base material kept dissolving.
Lead study author Shun Kashiwaya, a materials scientist at Linköping, turned to another piece of “100-year-old wisdom” to find the answer: a 1905 German article describing one of the reagent’s components, light-activated cyanide, which suggested applying the reagent in the dark could be key. After finding this vital clue, Kashiwaya says, “immediately I started to feel hopeful that exfoliation might work in darkness.”
He was right: the team managed to produce freestanding goldene flakes of about a tenth of a micron in area. The researchers also confirmed that the goldene has a higher binding energy than regular gold; this should help expand its ability to catalyze or jump-start chemical reactions. They plan to further explore goldene’s properties and ways to apply their method to other metals.
The new work “offers an exciting perspective for the development of 2D metals and understanding their properties,” says Yury Gogotsi, a materials scientist at Drexel University, who was not involved in the research. He adds that goldene “should be studied for potential catalysis and other applications.”