Like-Charge Particles Are Supposed to Repel—But From time to time They Attract
Experts believe they’ve cracked the lengthy-standing mystery of attraction between particles with a equivalent cost
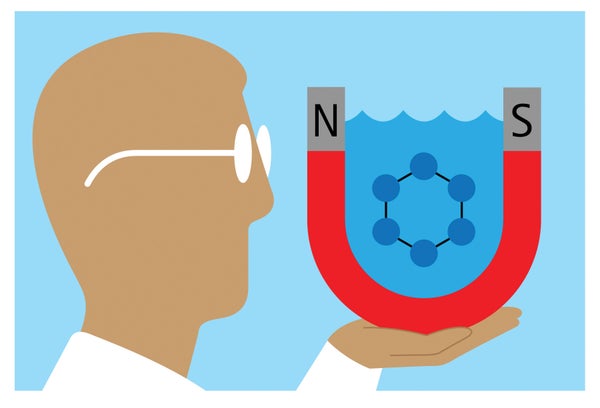
The actuality that like fees repel and opposites appeal to is fundamental electromagnetism. But for decades experts have from time to time built a counterintuitive, and controversial, observation: in the same way charged particles can from time to time also catch the attention of a single a further when dispersed in a liquid solvent these kinds of as drinking water or alcoholic beverages.
Researchers now propose in Mother nature Nanotechnology that this phenomenon arises from the solvent’s molecular character. The crew noticed that negatively billed silica particles pulled together and formed hexagonal clusters in h2o, and positively charged silica variants had been mutually attracted in liquor. Modeling h2o molecules’ behavior close to charged particles aided to reveal why.
In former experiments, scientists deemed a fluid to be a single continual compound, but this ignores the affect of its little atomic setting up blocks. H2o, for occasion, is created up of individual molecules that are dipoles—you can feel of them as having a lot more demand on a single aspect than on the other, like a battery, claims College of Oxford chemist and analyze co-author Madhavi Krishnan. And water molecules want to bond with other h2o molecules, so when they’re near a suspended particle they are likely to position their two marginally good hydrogen atoms toward the relaxation of the liquid and their slightly detrimental oxygen atom toward the particle.
On supporting science journalism
If you happen to be enjoying this posting, look at supporting our award-successful journalism by subscribing. By acquiring a subscription you are aiding to guarantee the foreseeable future of impactful tales about the discoveries and strategies shaping our planet today.
As negatively billed silica particles in h2o strategy just one another, they working experience an result termed charge regulation, whereby the repulsion between them pulls close by protons on to every single particle’s surface area, cutting down the particles’ damaging cost. That then weakens their repulsion from the water’s oxygen atoms, too, a phenomenon that intensifies as the silica particles go toward 1 a further. This change attracts the silica particles together from about a micron away.
The team observed the opposite outcome in alcohol, mainly because its molecules prefer to steer the other way at a particle’s area: positively charged particles suspended in liquor pull alongside one another alternatively. A solvent’s acidity also influences demand and therefore no matter if particles in it kind clusters.
Experts had been prolonged uncertain irrespective of whether these kinds of bizarre attraction was an experimental artifact or a real actual physical phenomenon, Krishnan claims. Critics have disputed previous observations of this result citing optical distortions, weak particle attraction, or hydrodynamic forces triggering particles to drift collectively. “This paper solves a mystery that has been out there for 20-plus years,” says Jay T. Groves, a chemist at the College of California, Berkeley. “It’s pretty thorough, and I believe [it’s] indisputable that this effect is a house of the solvent.”
This finding’s prospective takes advantage of are “limited to one’s have creativeness,” Krishnan says. The team’s long run function will investigate particle actions in other solvents, as effectively as purposes to fields these kinds of as biology: how molecules—many of which carry tons of electrical charge—organize themselves in cells.